The development of new and more efficient methods for the synthesis of pharmacologically important substructures as well as the synthesis and evaluation of specific molecules of potential medicinal interest are important goals in biomedical research. Our research spans each of these areas.
SC-INBRE Research
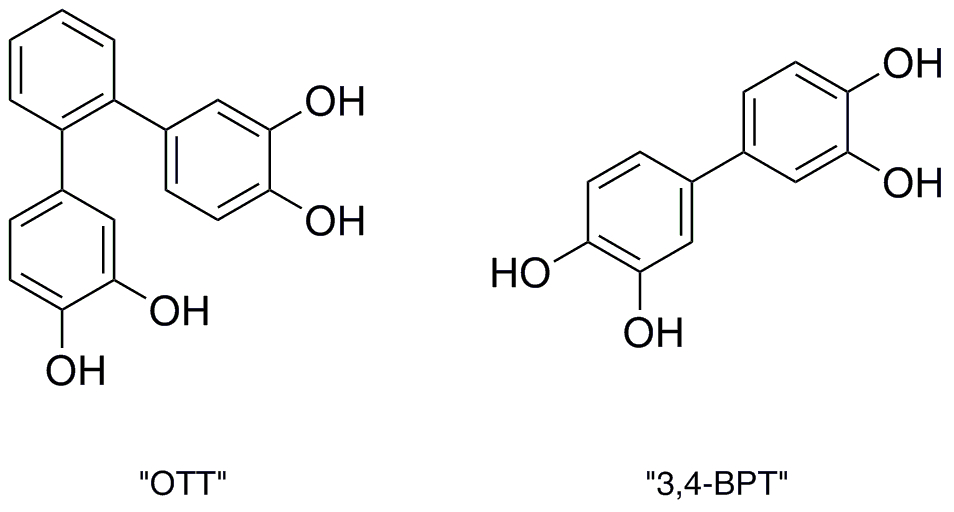
Synthesis and Evaluation of Terphenyl-Based Polyphenolic Compounds as Amyloid-beta Aggregation Inhibitors
Alzheimer’s disease (AD) is the most common form of dementia, affecting about 5 million people, and is the seventh leading cause of death. AD can be definitively diagnosed only in postmortem brains by the presence of plaques composed largely of fibrils consisting of aggregated amyloid-beta protein. While these plaques are the hallmarks of AD, the cognitive impairment associated with AD is thought to result from the formation of neurotoxic soluble oligomers of the amyloid-beta protein. Thus, the disruption of amyloid-beta self-association has been identified as a therapeutic goal for the potential treatment and/or prevention of AD. Based on recent data indicating that polyphenolic compounds are capable of inhibiting amyloid fibril formation in vitro, we are working collaboratively with Dr. Robin Lammi of the Winthrop University Chemistry Dept. on the synthesis of several biphenyl- and terphenyl-based polyphenolic compounds and evaluation of their activity against amyloid-beta aggregation.
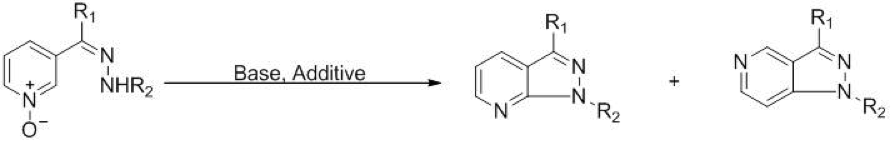
Synthesis of Fused Nitrogen Heterocycles by Intramolecular Amination of Azine N-oxides
The pyrazolopyridine ring system is recognized as a privileged substructure in medicinal chemistry. Many existing approaches to this ring system involve annelation of a pyrazole ring onto an existing pyridine by condensation with a suitably substituted hydrazine, but this requires the presence of a leaving group (most often a halogen) at the 2-position of the pyridine ring prior to cyclization. We imagined that this requirement could be eliminated by taking advantage of the electrophilicity of the 2-position of pyridine N-oxide (a strategy similar to the Reissert-Henze reaction). We have found that cyclization of pyridine N-oxide hydrazones proceeds under very mild conditions (room temperature) in the presence of an electrophilic additive and an amine base to give pyrazolo[3,4-b]pyridines and pyrazolo[4,3-c]pyridines in good overall yield. We are currently investigating the scope and limitations of this method including ways to control the regioselectivity of the cyclization and its application to the synthesis of other nitrogen-containing heterocycles.
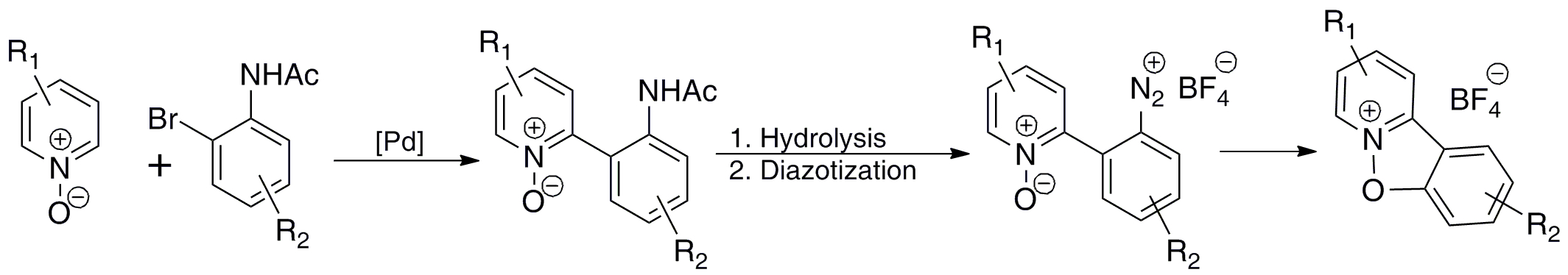
Synthesis of Novel Benzisoxazolopyridinium Salts
The benzo[4,5]furopyridine substructures are a common motif in a variety of biologically active compounds. Substances containing these structural units have been shown to exhibit activity against cancer, tuberculosis, osteoporosis, and HIV. Although considerable effort has been directed toward the synthesis of these structures, the related ring system, benzisoxazolo[2,3-a]pyridinium, has not attracted as much attention. Given the biological activity of the benzo[4,5]furopyridines and that the similar 2-arylisoxazolo[2,3-a]pyridinium salts have been reported to be useful anti-inflammatory agents and in the treatment of gastric hyperacidity, we became interested in developing a general synthesis of the benzisoxazolo[2,3-a]pyridinium ring system. We have found that direct arylation of pyridine N-oxide with o-bromoacetanilides, followed by hydrolysis of the resulting 2-(2-acetaminoaryl)pyridine N-oxides then diazotization and ring closure affords the desired benzisoxazolo[2,3-a]pyridinium salts. We are currently investigating the scope and limitations of this method and its application to the synthesis of other novel heterocyclic ring systems. We are also investigating the activity of these compounds as anti-cancer agents in a collaborative study with Dr. Takita Sumter (Winthrop University Chemistry Dept.).
Current Group Members (2011)
-
Chelsea Brennan
-
David Cantrell
-
Kourtland Haile
-
Jamie Murakami
-
Sarah Wicks
Former Group Members
- Brad Angel (2007-2008) – Veterinary student, University of Georgia. Honorable Mention in the 2008 SERMACS Undergraduate Oral Presentation Competition.
- Emily Conyers (2008-2009) – Quality Assurance Supervisor, Specialty Polymers, Chester, SC.
- Megan D'Angelo (2011-2012) – QC Technician, 3D Systems, Rock Hill, SC
- Ben Driscol (2006-2007)
- Lisa Kochurina (2007) – Pharm. D. graduate, Medical University of South CarolinaM
- Will Lominac (2010-2012)
- Joshua McClellan (2008-2009) – Chemist, Viance LLC, Charlotte, NC.
- Jeff Myers (2009-2012) – Graduate student, University of Virginia. Winner of the 2012 SC Academy of Science Dwight Camper Undergraduate Research Award. Winner of the Outstanding Oral Presentation Award in Chemistry and Chemical Engineering at the 2012 Big South Undergraduate Research Symposium.
- Darius Ollison (2010) – Associate Chemist, Nutra Manufacturing, Greenville, SC
- Monique Robinson (2006)
- Craig Stevens (2009-2012)
- Lee Varnedoe (2008-2009)
Group Publications (*undergraduate student):
- Lominac, W. J.*; D'Angelo, M. L.*; Smith, M. D.; Ollison, D. A.*; Hanna, J. M., Jr. Construction of Pyrazolo[3,4-b]pyridines and Pyrazolo[4,3-c]pyridines by Ring Closure of 3-Acylpyridine N-Oxide Tosylhydrazones. Tetrahedron Lett. 2012, 53, 906 – 909. (doi:10.1016/j.tetlet.2011.12.055, PMC3278155)
- Myers, J. T.*; Hanna, J. M., Jr. Palladium-Catalyzed Direct Arylation of Pyridine N-Oxide with 2-Bromoacetanilides. Synthesis of Benzisoxazolo[2,3-a]pyridinium Tetrafluoroborates. Tetrahedron Lett., 2012, 53, 612 – 615. (doi:10.1016/j.tetlet.2011.11.110, PMC3255091)
- Varnedoe, L. S.*; Angel, B. D.*; McClellan, J. L.*; Hanna, J. M., Jr. Pd/C-Catalyzed Cross-Coupling of Arenediazonium Salts with Potassium Aryltrifluoroborates. Lett. Org. Chem. 2010, 7, 1 – 6.
- Robinson, M. K.*; Kochurina, V. S.*; Hanna, J. M., Jr. Palladium-Catalyzed Homocoupling of Arenediazonium Salts: An Operationally Simple Synthesis of Symmetrical Biaryls. Tetrahedron Lett. 2007, 48, 7687 – 7690. (doi:10.1016/j.tetlet.2007.08.083)